

CAS number: 11113-63-6
Chemical formula: (CFx) n
Fluorocarbon ratio: 0.6-1.2
Grade and specification of Graphite fluoride
| Mark | FG05 | FG06 | FG07 | FG08 | FG09 | FG10 | FG1X |
| Appearance | black powder | gray-black powder | gray-black powder | gray-black powder | gray-white powder | white powder | white powder |
Average particle size | 20~50 μm | 10~20μm | 10~20μm | 10~20μm | 10~20μm | 10~20μm | 10~20μm |
| F/C ratio | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1 | ≥1.0 |
| resistivity | ~106Ω·m | ~107Ω·m | ~109Ω·m | ~1011Ω·m | ~1011Ω·m | ~1011Ω·m | ~1011Ω·m |
| Tap density | 0.80g/ml | 0.85g/ml | 0.89g/ml | 0.90g/ml | 0.91g/ml | 0.92g/ml | 0.93g/ml |
Typical SEM image of flake Graphite fluoride

Typical SEM image of flake Graphite fluoride(powder)

Typical SEM image of flake Graphite fluoride(Synthetic)

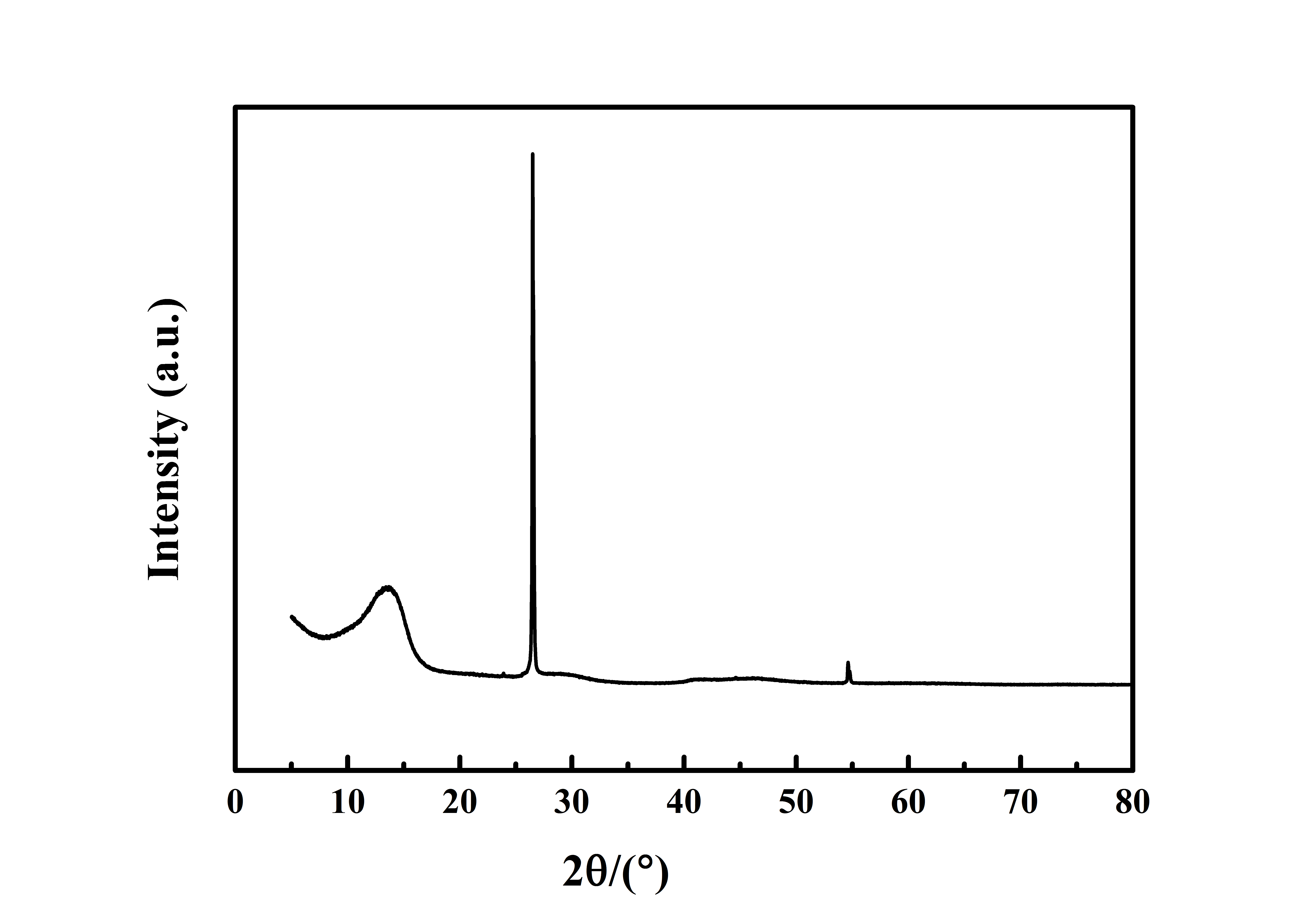
Typical XRD analysis of flake Graphite fluoride
Graphite fluoride- Profile
Graphite fluoride is a kind of graphite interlayer compound prepared by direct reaction of carbon and fluorine. Its chemical structure can be expressed by (Cfx) n. Where X is an indefinite value with a size of 0 < X < 1.25.
Graphite fluoride is a new type of functional material, belonging to graphite deep processing products. It has a very low surface free energy, and its thermal stability and chemical stability is good, so it has a unique lubricity, hydrophobic oleophobic, and its chemical and thermal stability is also very good, so it can be used as a solid lubricant, waterproof and oil-proof agent, antifouling agent, etc. In addition, Graphite fluoride can be used as a battery active substance, it can increase the storage life of the battery, and can be used to manufacture high-energy primary batteries. Because Graphite fluoride has many excellent properties, it is widely used in military, aviation, aerospace, metallurgy, mechanical and electrical, chemical and other fields.
Graphite fluoride - Structure
Due to the layered structure of graphite, the carbon atom spacing within the layer is 1.4A, which is firmly linked together by covalent bonds, and the carbon atom spacing between the layers is 3.35A, which only has a weak van der Waals force, so it is easy to insert heterogeneous substances between the graphite layers to form graphite interlayer compounds. When the graphite layer is fluorine, the graphite interlayer compound that can be formed is Graphite fluoride. Fluorinated inks have different characteristics with the difference of fluorocarbon ratio, but only fluorinated inks (CFx) with fluorocarbon ratio of no less than 1 have good chemical and hot pressing stability. In this Graphite fluoride, fluorine forms A covalent bond with the 2Pz electrons of carbon atoms, the spacing of carbon atoms in the layer increases to 1.52 A, and the layer is bent, thus losing the conductivity of graphite and becoming an insulator. At the same time, due to the electronegativity of fluorine atoms, the interlayer carbon atom spacing extends from 3.35A to 7.08A, resulting in greatly reduced interlayer energy and significantly improved lubrication performance. Therefore, Graphite fluoride is a typical molecular crystal.
Graphite fluoride's formation mechanism

Graphite fluoride -Physicochemical property
Fluorinated ink is a white solid powder substance with a density of 2.58×103 kg/m3. It has the properties of low surface energy, high lubricity and electrical activity, and smaller thermal neutron absorption area than other sealing materials. The properties of fluorinated inks vary with the ratio of carbon to fluorine in the molecular formula. CF (1-1.25) is called high-fluorinated graphite CF (0.5-0.99) is called low-fluorinated graphite color with the increase of fluorine content, from gray black to Snow White, high-fluorinated graphite has excellent thermal stability, is an electrical and thermal insulator, not subject to strong acid and alkali corrosion, lubrication performance more than MoS2 and flake graphite, test proved, At any temperature, its wear life is better than MoS2 as an additive for lubricating wax, which can significantly increase the supporting load of the parts and reduce the surface temperature of the lubricating parts. The appearance of low fluorinated fossil ink is gray and black, and the thermal stability is poor. Generally, the use of fossil graphite fluoride without lubricant has a large wetting contact Angle and a low surface energy.
Graphite fluoride-Application fields
1.High-energy lithium fluoride Li/ (CF) n battery cathode material (fluorocarbon ratio 0.6) : is a new type of high-energy lithium ion battery, with fluorinated fossil ink or fluorinated coke as a new positive electrode material, lithium as a negative electrode, with organic solvents as the medium. Among all the positive electrode materials of lithium batteries, fluorocarbon has the highest mass specific capacity, so the lithium monofluorocarbon battery with fluorocarbon as the positive electrode material has the unique advantages of large specific energy, wide temperature range and stable operating voltage. The test results show that the specific energy of the lithium battery with fluoro-fossil ink as a positive electrode is increased by nearly 30%, and it can also discharge stably at the rate of 3C, and its specific power characteristics are improved by an order of magnitude, which greatly improves the electrochemical performance of the lithium battery.
Lithium fluorocarbon power supply is based on stainless steel as a container, lithium metal as a negative electrode, carbon fluoride as a positive electrode, lithium inorganic salts and organic solvents as an electrolyte, glass fiber as a diaphragm of the primary lithium primary battery, with high voltage (3V), high capacity, small size advantages, specific energy reached 250wh.kg and 500wh.L above, the annual capacity is reduced to 2%.
This product has the characteristics of small size, long storage life, safe use and wide operating temperature range, and the battery can work normally at -40℃~+125℃. These products are usually used in special applications where lithium manganese batteries cannot be used, such as automotive tire pressure meters (TPMS), industrial control motherboards, set-top boxes, smart meters, smart water meters, smart gas meters, gas pipeline data transmission, military applications and other applications. In particular, military applications and automobile tire pressure gauges are the most widely used and have the greatest demand.
2.Lubricating oil anti-wear additives (fluorocarbon ratio 1.0) : Graphite fluoride molecular groups can quickly spread film on the surface of the internal friction pair of the engine, and show strong anti-wear performance on the metal friction pair, fully extend the engine life, save fuel, improve power, and the oil change cycle can reach 50,000 kilometers.
3.In the military field: Graphite fluoride can be used in the secondary explosion of the missile, the energy released by the missile explosion can promote the decomposition of Graphite fluoride, which releases a lot of energy and explodes more violently than the first explosion of the missile.
4.Other uses: added to the paint can improve the coating performance; When the plywood is produced, it is used to treat the wood in advance, which can reduce the penetration of the binder to the wood and save raw materials. Used for image recording and reproduction, chromatographic analysis of materials, etc.